INTRODUCTION OF MICROSCOPIC STRUCTURE OF SKELETON MUSCLES
In this topic, we will discuss the features of skeleton muscles and the microscopic structure of skeletal muscles in detail.
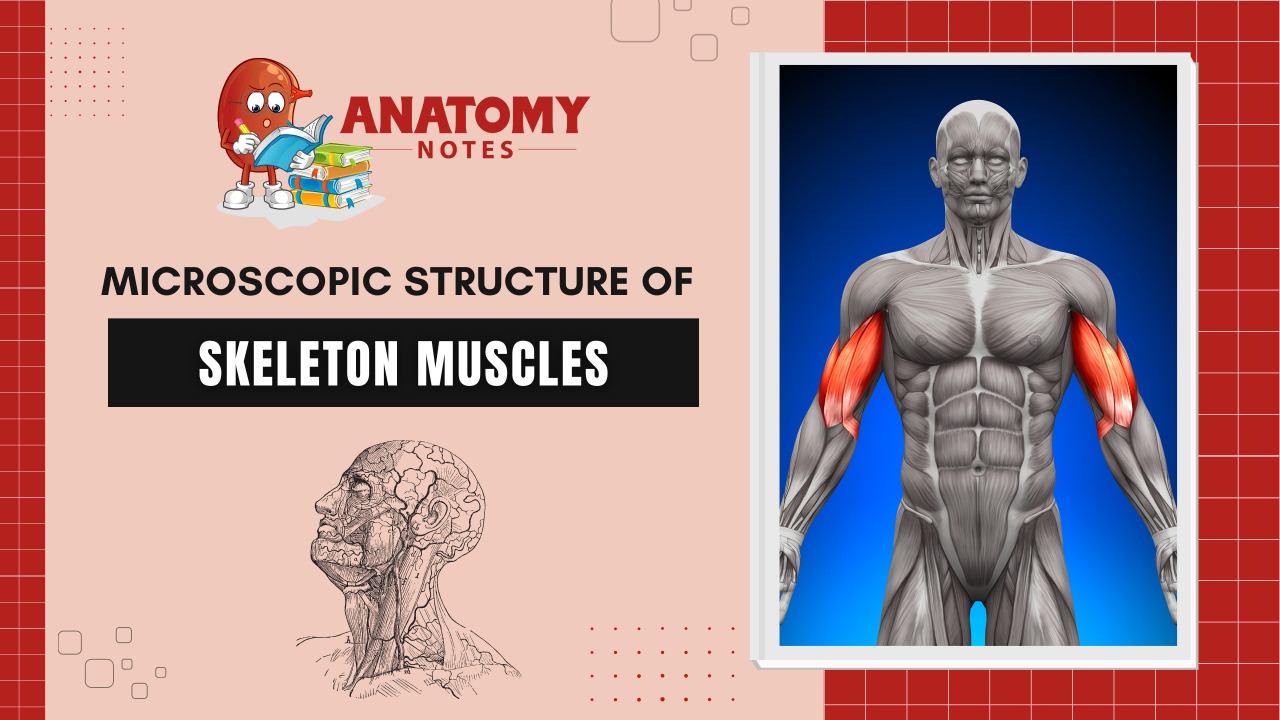
Skeletal muscles are striated and voluntary. It is the most common muscle tissue. It consists of long, parallel multinucleate cells bundled together by collagenous sheaths and through this regular organization allow the skeletal muscles to generate powerful contractions, along with a power output up to 100 watts per kilogram of tissue.
This organization is a limited contractile range: skeletal muscle can shorten by only 30%. If a larger range of movement is required, it must be achieved through the amplification provided by lever systems.
Also Read: Cartilage – Introduction, Structure, Formation And Types Of Cartilage
https://anatomynotes.org/skeletal-system/cartilage/cartilage-introduction-structure-formation-and-types-of-cartilage/
Skeletal muscle is innervated by somatic motor nerves and is sometimes referred to as voluntary muscle because its contractions are often initiated under conscious control. However, this is a mislead-in term because skeletal muscle is involved in many movements, such as breathing, blinking and swallowing, which are often initiated at an unconscious level.
MICROSCOPIC STRUCTURE OF SKELETON MUSCLES
The skeletal muscle fiber is known as enormous multinucleate cells and it develops through the fusion of each myoblast. These myoblasts are long, cylindrical structures with consistent size within the muscles. The size of myoblast may vary in different muscles from 10 to 100 µm in diameter, and from a few millimeters to many centimeters in length.
The plasma membrane which is called sarcolemma surrounds the cytoplasm of each fiber and sarcoplasm. When sarcoplasm comprises into a piece of contractile machinery and formed myofibrils of 1-2 µm in diameter, which extend the length of the fiber.
The thin transparent rim of sarcoplasm occupied by numerous euchromatic, oval nuclei between the myofibrils and sarcolemma, and also in the region of the neuromuscular junction.
A muscle fiber may contain several hundreds of nuclei along its entire length. And in between the sarcolemma and surrounding basal lamina, there is myogenic satellite cells are present.
SARCOMERE OF SKELETON MUSCLES
Myofibrils are regularly packed too tightly to be visible by light microscopy, their presence can be detected by transverse stripes across the tissue. Cross-strikes can be performed more effectively using special stains or under polarized light, ranging from light, isometric I-bands to dark, anisotropic A-bands (which are bisected and the plane of polarized light Rotate vigorously) can separate (rotate the plane) polarized light to a negligible degree).
Also Read: Muscular system – Types of muscles, characteristics & functions
Muscular system – Types of muscles, characteristics & functions
In the transverse section, skeletal muscle fibers are usually polygonal and their sarcoplasm often has a striated appearance, as transversely sectioned myofibrils resolve as dots. The packing density of muscle fibers varies from low (in the outer muscles of the larynx) to high (in the muscle group that enhances trauma).
The most detail is detected by transmission electron microscopy. Myofibrils, about 1 μm in diameter, are the dominant ultrastructural feature. In longitudinal segments, they appear as ribbons that are interrupted at regular intervals by thin intersecting transverse lines, corresponding to discs in the original cylindrical structure.
These are the Z-lines or, more precisely, the Z-disc (Zwischenscheiben = interval disc) which divide each myofibril into a linear series of repeating contraction units, sarcomeres. A sarcomere is typically 2.2 μm long in resting muscles. At high power, it can be seen consisting of two types of filament, thick and thin, arranged in regular arrays. Thick fibers, which are about 15 nm in diameter, are mainly made of myosin. Thin filaments, which are 8 nm in diameter, are mainly composed of actin.
Thick and thin filament arrays form partially overlapping structures in which the electron density (as seen in the electron microscope) varies according to the amount of protein. The A-band consists of coarse fibers, together with a length of thin fibers, which are interrelated, and thus coarse fibers at the end.
The middle of the A-band, the palate region, which is not penetrated by thin filaments, is called the H-zone (Hale = light). At their centers, thick fibers are joined together by material transversely attached. M-line (Mittelscheibe = middle []] disc), which is visible in most muscles. The I-band consists of adjacent parts of two neighboring sarcomeres in which the thin filaments are not overlapped by thicker fibers.
Thin filaments of the surrounding sarcomeres are anchored in the Z-disk, which bisects the I-band. The high degree of organization of thick and thin filaments is equally evident in transverse sections. The thick myosin filaments form a hexagonal lattice.
In regions where they overlap thin filaments, each myosin filament is surrounded by six actin filaments at the trigonal points of the lattice. In the I-band, the thin filament pattern changes from hexagonal to square as the filaments approach the Z-disk, where they are incorporated into a square lattice structure.
The bound appearance of individual myofibrils is a function of the regular alternation of thick and thin filament arrays. The size of myofibrils places them at the limit of resolution of light microscopy; Cross-strips appear only at that level because of the alignment of the register of A- and I-bands in the surrounding myofibrils across the width of the entire muscle fiber.
In suitably stained resting materials, the A-, I- and H-bands are quite distinct, while the Z-disc, which is a key feature of electron micrographs, is thinner and much less specific in a light microscope, and M – Lines cannot be resolved.
MUSCLES PROTEIN OF SKELETON MUSCLES
Myosin, a coarse fibrous protein, accounts for 60% of the total myofibrillar protein and is the most abundant contracted protein. The thick filament of skeletal and heart muscle is 1.5 μm long. His creation liquid sustanon 250 is described from myosin heavy and light series assemblies. Other components of myosin, the regulatory proteins tropomyosin and troponin play a major role in the control of contraction.
Actin is the next most abundant contractile protein and accounts for 20% of the total myofibrillar protein. In its filamentous form, F-actin, it is the major protein of thin filaments. The components of a thin filament assembly have a number of congenital myopathy from gene mutations.
The third type of long sarcomeric filament connects the coarse fibers to the Z-disk and is formed by the giant protein, titin, which has a molecular mass in the millions. The molecules of a single titin span half the sarcomere between the M-lines and the Z-disk into which they are inserted.
They have a tethered portion in the A-band, where they are joined by thick fibers as an M-line and an elastic part in the I-band. The elastic properties of titin are characterized by passive stretching of pulled muscle fibers and endowed with elastic repetition.
Many proteins that neither shrink nor regulate are responsible for the structural integrity of myofibrils, particularly their regular internal arrangement. The Z-disk, a component of α-actinin, is a rod-shaped molecule that anchors the plus-end of the actin filament from the surrounding sarcomere to the Z-disk.
Nebulin inserts into the Z-disk, which is joined by thin filaments and controls the length of actin filaments. Desmin, an intermediate filament protein characteristic of muscle, innervates myofibrils on the Z-disk and, together with the linking molecule purine, forms a mesh that connects myofibrils within muscle fibers and together with sarcolemma. Myosin places myosin filaments in their regular lattice arrangement in the region of the M-line.
Dystrophin is limited to the periphery of the muscle fibers, which are close to the cytoplasmic face of the sarcolemma. It binds actin intracellularly and is also associated with a large oligomeric complex of glycoproteins, the dystroglycan/sarcoglycan complex that stretches the membrane and specifically links with merosin, the α2-laminin of the basal lamina of the muscle. isoform. It stabilizes muscle fibers and transmits internally generated forces upon contraction in the outer matrix.
Dystrophin is the product of genes affected in Duchenne muscle development, a fatal disorder that develops when a mutation of a gene leads to the absence of a protein.
A mild form of the disease, Baker muscle development, is associated with a reduced size and/or abundance. Female carriers of Duchenne muscular dystrophy (asymptomatic for mutant genes) may also have mild symptoms of muscle weakness.
At around 2500 kb, the gene is one of the largest discovered so far, which may have a high mutation rate (about 35% of cases new mutations) of Duchenne muscle development.
Other muscle dystrophies may include deficiencies in proteins functionally associated with dystrophin, such as the dystroglycan/sarcoglycan complex or α2-laminin; They may also be the result of mutations in proteins of the inner nuclear membrane.
OTHER SACROPLASMIC STRUCTURES OF SKELETAL MUSCLES
Although myofibrils are the dominant ultra-structural feature, skeletal muscle fibers contain other organs required for cellular function. The ribosome, the Golgi apparatus, and the mitochondria lie around the nucleus, between myofibrils and sarcolemma, and, to a lesser extent, between myofibrils.
Mitochondria, lipid droplets and glycogen provide the metabolic support required by activated muscle. The mitochondria are elongated and their cristae are closely packed.
The number of mitochondria in an adult muscle fiber is not fixed, but can increase or decrease quite easily in response to sustained inactivation. Spherical lipid droplets of about 0.25 μm are evenly distributed in the sarcoplasm among myofibrils.
Introduction to Histology – Applications & Importance
Introduction to Histology – Applications & Importance
They represent a rich source of energy that can only be tapped by oxidative metabolic pathways; They are therefore more common in fibers that have high mitochondrial content and good capillary blood supply.
Small clusters of glycogen granules are spread between myofibrils and thin filaments. During brief bursts of activity, they provide an important source of anaerobic energy that does not depend on the flow of blood in muscle fibers.
Tubular infiltration of sarcolemma between myofibrils in a transverse plane at the boundary of each A-band. The lumina of these transverse (T) ducts are thus in continuity with outer space.
At the ends of the muscle fibers, where force is transmitted to adjacent connective tissue structures, sarcolemma is tied into multiple finger-like projections that strengthen the junction region by increasing the area of engagement.
The sarcoplasmic reticulum (SR) is a specialized form of the smooth endoplasmic reticulum and forms a plexus of the anastomosing membrane cistern that fills more space between the myofibrils.
The cisternae extend into the large sac, junctional sarcoplasmic reticulum or terminal cisternae, where they come into close contact with the T-tubules, forming a structure called triads.
SR membranes have calcium-tricep pumps that carry calcium ions to the terminal cistern, where ions bind to calsequestrin, a dense storage grain containing a protein with a high affinity for calcium.
In this way, calcium can be accumulated and retained in the terminal cistern at a much higher concentration than elsewhere in the sarcoplasm. Ca2 + -release channels (Ryanodinereceptors) are mainly concentrated in the terminal cistern and form one-half of the feet or ars columns of the junction that bridge the SR and T-tubules at the tip.
The other half of the junction leg is the T-tubule receptor that constitutes a voltage sensor.
Learn More: Anatomy and physiology
Frequently Asked Questions (FAQs)
What are the structures and functions of the skeletal muscle?
Skeletal muscles are composed of muscle fibers arranged in bundles called fascicles. They are attached to bones by tendons and contract to produce movement. Skeletal muscles are under voluntary control and provide support, stability, and movement to the body.
What are the 4 structures of skeletal muscle?
The four structures of skeletal muscle are muscle fibers, myofibrils, sarcomeres, and motor units. Muscle tissue comprises long and cylindrical cells called muscle fibers. Myofibrils are the contractile units within muscle fibers, made up of actin and myosin filaments. Sarcomeres are the repeating units of myofibrils, responsible for muscle contraction. Motor units are composed of a motor neuron and the muscle fibers it innervates, and are responsible for initiating muscle contraction.
What is the structure of the muscle?
The structure of a muscle can be described as a hierarchy of different levels of organization. At the macroscopic level, muscles are made up of many muscle fibers, which are long, cylindrical cells that run parallel to each other. The perimysium is a connective tissue layer that encloses bundles of muscle fibers known as fascicles. Fascicles are then bundled together by another layer of connective tissue called the epimysium, which forms the outer layer of the muscle.
At the microscopic level, each muscle fiber contains many myofibrils, which are the contractile units of the muscle. Myofibrils are composed of repeating units called sarcomeres, which contain the proteins actin and myosin. Sarcomeres are responsible for muscle contraction and give the muscle its striped appearance under a microscope.
What are examples of skeletal muscles?
Skeletal muscles are the muscles that attach to the bones and produce movements of the body. They are also called striated muscles because of their striped appearance under the microscope. Some examples of skeletal muscles in the human body include the biceps brachii, triceps brachii, deltoid, pectoralis major, rectus abdominis, quadriceps femoris, hamstrings, gastrocnemius, and soleus. These muscles are responsible for movements such as bending and extending the arm, lifting objects, rotating the shoulder, flexing the trunk, extending the leg, and planar flexing the foot.
How many are skeletal muscles?
There are over 650 skeletal muscles in the human body. These muscles vary in size and shape, and work together to produce movement, maintain posture, and stabilize joints. Some of the largest skeletal muscles in the body include the gluteus maximus in the buttocks, the quadriceps in the thighs, and the latissimus dorsi in the back. The smallest skeletal muscles are found in the middle ear and are involved in the process of hearing.
What are the 3 structures of muscles?
The three structures of muscles are:
Muscle fibers: Long, cylindrical cells that make up the muscle tissue and are capable of contracting.
Fascicles: Bundles of muscle fibers that are surrounded by a layer of connective tissue called perimysium.
Muscle: A collection of fascicles bound together by another layer of connective tissue called epimysium. The epimysium forms the outer layer of the muscle and connects it to tendons, which attach the muscle to bones.